ground state electron configuration|The Electron Configurations of Atoms : Bacolod We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams . Post screenshot's and/or tell us about the unintended effects funnies bloopers etc. that you've encountered and/or created using GG.There are different ways to create raffle tickets, but the commonest method is the use of enter to win templates. You may want to get ideas from raffle ticket samples before you decide on the designs you want. Follow these steps to create your raffle ticket designs. Step 1 – Prepare the raffle ticket template
PH0 · The Electron Configurations of Atoms
PH1 · Ground State Electron Configuration
PH2 · Electron configuration
PH3 · Electron Configuration
PH4 · Ch 1 : Orbital Fillling & Electron configurations
PH5 · 6.4 Electronic Structure of Atoms (Electron Configurations)
PH6 · 2.6: Electron Configurations
PH7 · 1.9A: Ground State Electronic Configurations
PH8 · 1.3 Atomic Structure: Electron Configurations
14 talking about this
ground state electron configuration*******Learn how to write and understand ground state electron configurations using the Aufbau principle, quantum numbers, orbitals, and rules. See examples, .We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. Orbital diagrams .ground state electron configuration The Electron Configurations of Atoms This chemistry video tutorial explains how to write the ground state electron configuration of an atom / element or ion using noble gas notation and how to f. The ground-state electron configuration tells us the lowest energy state that an atom can have. This tells us the unexcited state of the atom, which is the s.Learn how to predict the lowest-energy arrangement, or ground-state electron configuration, of an atom by following three rules: Aufbau principle, Pauli exclusion principle, and .Learn how to determine the ground state electron configuration of an atom using a periodic table. See examples of carbon and aluminum and their orbital energy diagrams.By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state electron configuration and orbital diagram .Learn how to write the distribution of electrons in atomic or molecular orbitals for atoms and molecules. Find out the notation, rules, and examples of electron configurations, .
In order to write its electron configuration, we must first determine (from Figure 5.9) that the 2s sublevel is next higher in energy after the 1s sublevel. Therefore, the .
ground state electron configuration The electronic configuration of cations is assigned by removing electrons first in the outermost p orbital, followed by the s orbital and finally the d orbitals (if any more electrons need to be removed). .In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar] 4s 1 3d 5 rather than the predicted [Ar] 4s 2 3d 4. Transition Metals with an Oxidation State. In the ground state, the electron configuration of the transition metals follows the format, ns 2 nd x. As for the electron configuration for transition metals that are charged (i.e. Cu +), the electrons from the s orbital will be moved to the d-orbital to form either ns 0 nd x or ns 1 nd x.
The Electron Configurations of Atoms Electron configurations of elements beyond hassium (element 108) have never been measured; predictions are used below. As an approximate rule, . subsection Electron Configuration of Neutral Atoms in the Ground State. 91 Pa : [Rn] 5f 2 (3 H 4) 6d 7s 2; 92 U : [Rn] 5f 3 (4 I o 9/2) 6d 7s 2; The electron configuration states where electrons are likely to be in an atom. If you don’t have a chart, you can still find the electron configuration. Use the element blocks of the periodic table to find the highest electron orbital. Alternatively, remember group 1 (alkali metals) and group 2 (alkaline earth metals) are s-block, groups .
The ground state electron configuration of arsenic is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 3. This electron configuration shows that the last shell of arsenic has five electrons. Therefore, the valence electrons of arsenic are five. Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
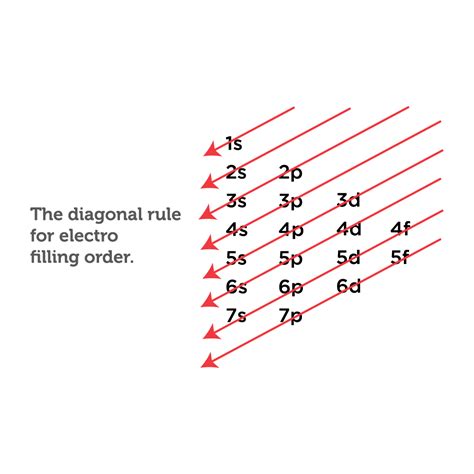
Experimentally, we observe that its ground-state electron configuration is actually [Kr]5s 1 4d 4. We can rationalize this observation by saying that the electron–electron repulsions experienced by pairing the electrons in the 5s orbital are larger than the gap in energy between the 5s and 4d orbitals. There is no simple method .In several cases, the ground state electron configurations are different from those predicted by Figure \(\PageIndex{1}\). Some of these anomalies occur as the 3 d orbitals are filled. For example, the observed ground state electron configuration of chromium is [Ar]4 s 1 3 d 5 rather than the predicted [Ar]4 s 2 3 d 4 . The ground state electron configuration of vanadium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 3 4s 2. In the vanadium ground-state electron configuration, the three electrons of the 3d orbital are located in the d xy, d yz, and d zx orbitals. We already know that the d-subshell has five orbitals.

The ground state electron configurations contribute to a fundamental understanding of the periodic table and will later be used to understand different atomic properties. The Aufbau Principle. The Aufbau Principle (also called the building-up principle or the Aufbau rule) states that, in the ground state of an atom or ion, electrons fill . The ground state electron configuration of chromium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 1. This electron configuration shows that the last shell of chromium has an electron and the d-orbital has a total .
The Aufbau Principle states that, in a ground state electron configuration, the lowest energy orbitals are filled with electrons first. For example, using carbon as the example again, the 1s .Figure 3.29 shows the lowest energy, or ground-state, electron configuration for these elements as well as that for atoms of each of the known elements. Figure 3.29 This version of the periodic table shows the outer-shell electron configuration of each element. Note that down each group, the configuration is often similar.
Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation .
The ground-state electron configuration tells us the lowest energy state that an atom can have. This tells us the unexcited state of the atom, which is the s. The ground state electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3. We already know that the p-subshell has three orbitals. The orbitals are p x, p y, and p z and each orbital can have a maximum of two electrons. Phosphorus ground-state electron configuration and orbital diagram. The ground-state electron configuration of lead is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10 6s 2 6p 2. In the tin ground-state electron configuration, the last electrons of the 6p orbital are located in the 6p x and 6p y orbitals. We already know that the p-subshell has three orbitals.
Cheltenham Gold Cup 2022 Ante-Post Odds as HorseRacing.net take an early look at the Betting Market for the feature race on Day 4 at the 2022 Cheltenham Festival. . Third behind Al Boum Photo in the 2020 Cheltenham Gold Cup, Lostintranslation has lost his way a little in recent times, but he showed when bounding to victory in the 1965 Chase .
ground state electron configuration|The Electron Configurations of Atoms